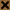- Potential problems that may arise when mixing different kinds of engine oil
- Reaction between additives
- Sedimentation and precipitation of additives
- Reaction between additives
- Additives mixed to engine oil
- A blanced formulation of detergent dispersant, anti-corrosion agent, anti-oxidant, viscosity index improver (VII), antirust agent, pour point depressant (PPD), and friction modifier (FM).
- Additives are acidic, basic (alkaline), and a fraction are neutral
- Reaction mainly occurs due to the collapse in balance between acidic and basic additives
- Example of reactions between additives
- The basic Ca-Sulfonate which is included in detergent dispersants reacts with fatty acids from friction modifiers or organic acids from antirust agents to produce calcium salts, resulting in decreased or loss of perfomance
- Ashless detergent dispersants and ZnDTP (antioxidants/antiwear agents) which are formulated in engine oil react with each other to produce complex compounds
- Charateristics of the reaction
- Cautions is required as the organic reaction of a non-aqueous system is like an aqueous solution reaction in which water is used as solvent (mediator), where results are not yielded quickly and clearly as reaction rates are slow and changes to reation sstem are revealed after an extended period.
- Sedimentation and precipitation of additives
- Highly refiend oil is recently being used in engine oil formulas
- Advantage: Excellent stability and perfomance of additive
- Disadvantage: Solubility is inferior to conventional oil ( Group-I). As the ability to hold additives in oil is reduced, there is high probality of precipitation and sedimentation of highly polar friction modifier.
- Mixing of diferent engine oils with varying purposes results in higher probability of sedimentation and precipitation of additives due to differences in solubility.
- Mixture with used oil
- Vanish and sludge (oxide) precipitants resulting from deterioration which are found in oil or inside engines, have a very small amount of peroxide, which is immersed into new oil during engine oil changes.
- Peroxide accelerates the oxidative deterioration of oil as it acts as the core material involved in the oxidative chain reaction
- Antioxidants in new oil react with such peroxides but are consumed at the same time. If the amount of peroxide exceeds the treatment capacity of the antioxidant, oxidative deterioration due to immediate chain reactions start even in freshly exchanged new oil.
- When eexchanging oil, removal of fluishing of the used oil is insufficient. Vanish and sludge (oxide) attached inside the engine must be completely removed.